🇲🇽 NOM-051 in Mexico: it’s not just for Mexican companies
- Futuro Imperfecto

- Dec 12, 2025
- 3 min read
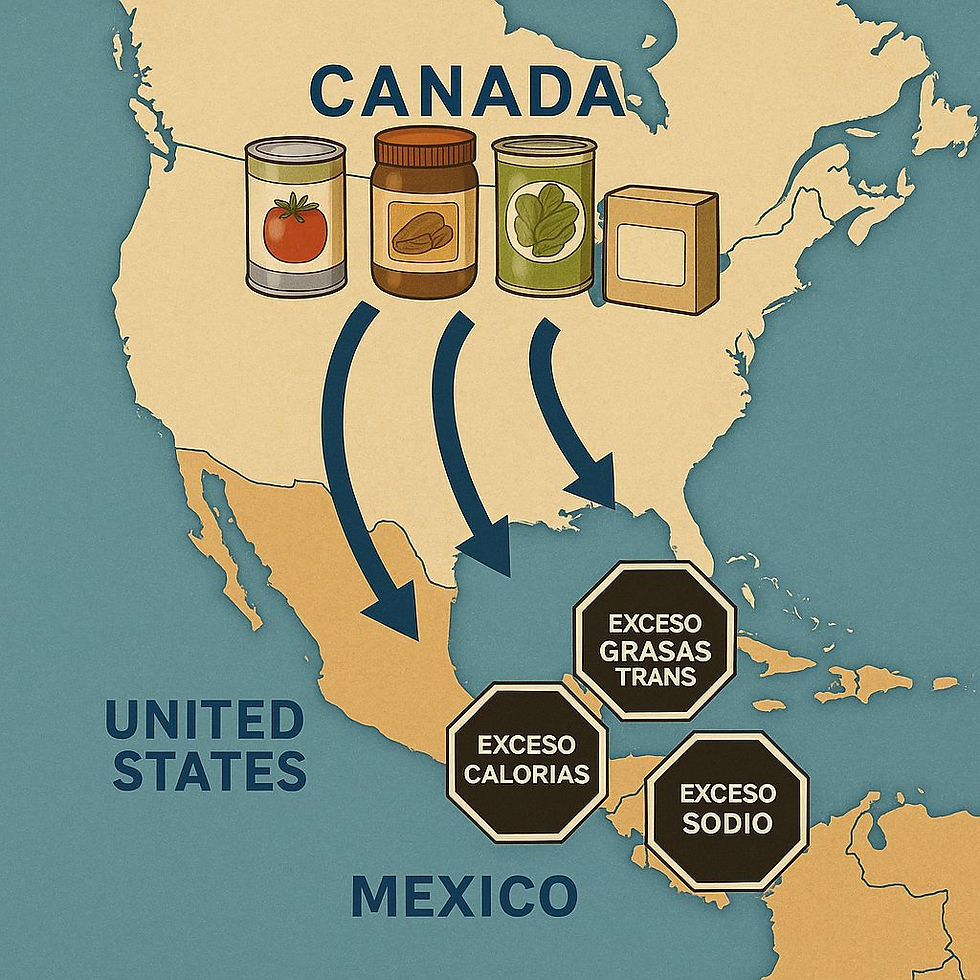
There’s still a persistent idea that Mexico’s food labeling regulations are a “local issue.” They aren’t.
If a company wants to sell food or beverages in Mexico, whether it’s in Montreal, Toronto, São Paulo or Singapore, NOM-051 is also their regulation. Not complying simply means not entering the market.
Why Canadian companies (and any international manufacturer) should care
NOM-051-SCFI/SSA1-2010 applies to all prepackaged foods and beverages sold in Mexico, regardless of country of origin. It includes:
Front-of-pack warning seals
Mandatory nutrition declaration
Precautionary statements
Strict rules for nutrition and health claims
(Yes, the full package. And yes, importers must comply too.)
The size of the market at stake
Mexico is one of the largest CPG markets in the Americas*:
USD 110 billion in total CPG market size (2024)
USD 78.4 billion in food retail alone (2023)
USD 6.08 billion in food packaging (2024), growing due to regulation and convenience trends.
This isn’t a “Latin American niche.” It’s a massive market that requires technical compliance starting at the product-design stage.
Phase 2 and Phase 3: where things stand now
The 2020 update to NOM-051 established three implementation phases.
Phase 2 (currently in effect)
Started on October 1, 2023
In this phase, if you add a critical nutrient, only the added nutrient is evaluated (for example, if you add only sodium, only sodium is assessed even if sugars are naturally high)
In 2025, the Official Gazette published an agreement extending Phase 2 until December 31, 2027, and moving the start of Phase 3 accordingly
Phase 3 (effective January 1, 2028)
This is where the real challenge begins:
If a single critical nutrient is added (sugars, fats, saturated fats, trans fats or sodium), the evaluation will consider the total content of all critical nutrients, including naturally occurring ones
This increases the likelihood of warning seals and restricts allowed claims
Reformulation will no longer be optional for many categories
What this means for companies outside Mexico (especially Canadian ones)
The extra time is not an excuse to wait. The extension of Phase 2 to 2027 doesn’t eliminate Phase 3; it simply gives you more room to adjust formulations, claims and packaging.
Planning only a label change is not enough. Under Phase 3, reformulation may be the only path to reducing warning seals, especially in sugar- or sodium-heavy categories.
Exporting to Mexico requires designing with compliance in mind. “Fixing the label at the end” won’t work. You need:
Canada–Mexico coordination is essential. Trade agencies and technical guides for Canadian exporters already emphasize that NOM-051 applies fully to imported products and recommend working with accredited Mexican verifiers and specialized regulatory teams.
How we’re supporting this at Elytra Biomaterials
At Elytra, we are developing Agentic AI/ML Systems specifically designed to support R&D, Quality and Regulatory teams in:
Automated compliance analysis for NOM-051, NOM-086 and NOM-131
Simulation of reformulation scenarios and changes to the nutrition panel
Predictive evaluation of front-of-pack warning seals under Phase 2 and Phase 3
Automated generation of print-ready nutrition facts tables
And because no one should navigate Mexican regulation alone, we’re working in partnership with Akshita Puri from Texave International, a firm with direct expertise in regulatory processes, verification, and exporting to Mexico.
Technology and regulatory insight in the same toolkit. No last-minute scrambling.
In summary
Mexico is a huge and accessible CPG market for global companies—provided they meet regulatory requirements.
We are in Phase 2 until 2027; Phase 3 in 2028 fundamentally changes how critical nutrients are evaluated.
Companies that prepare now will have a competitive advantage later.
And yes, Mexican regulation can become a strategic design advantage when integrated early, not patched at the end.
*Sources:
Grand View Research – Mexico Consumer Packaged Goods Market Report (2024)
USDA Foreign Agricultural Service – Mexico Retail Foods Annual Report (2024)
Grand View Research – Mexico Food Packaging Market Report (2024)



Comments